Contents
- I. Introduction
- II. Understanding Antibiotic Resistance
- III. Impact of Antibiotic Resistance
- IV. Common Antibiotic-Resistant Infections
- V. Factors Contributing to Antibiotic Resistance
- VI. Strategies to Combat Antibiotic Resistance
- VII. Importance of Proper Antibiotic Use
- VIII. Role of Healthcare Providers in Combating Antibiotic Resistance
- IX. Antibiotic Resistance and the Environment
I. Introduction
Welcome to the world of antibiotic resistance, where the effectiveness of our most powerful drugs is diminishing. Antibiotic resistance is a global health crisis that threatens to undo decades of medical progress. In this article, we will explore the dangers of antibiotic resistance and discuss strategies to combat this growing problem.
Antibiotics have been a game-changer in the field of medicine, saving countless lives by fighting off bacterial infections. However, the overuse and misuse of antibiotics have led to the emergence of antibiotic-resistant bacteria. These superbugs are not affected by the drugs that once eradicated their less resistant counterparts.
The consequences of antibiotic resistance are dire. Simple infections that were once easily treatable can now become life-threatening. Common medical procedures such as surgeries, chemotherapy, and organ transplants become riskier due to the increased likelihood of infection. The economic burden is also significant, with healthcare costs skyrocketing and productivity losses mounting.
So, how did we get here? The misuse of antibiotics in both human and animal healthcare is a major contributing factor. Overprescribing antibiotics, not completing the full course of treatment, and using antibiotics in animal feed for growth promotion are all practices that have fueled the rise of antibiotic resistance.
In the next sections, we will delve deeper into the causes and consequences of antibiotic resistance. We will also explore strategies to combat this issue, including the responsible use of antibiotics, the development of new drugs, and the importance of public awareness and education. Together, we can work towards a future where antibiotics remain effective and continue to save lives.
II. Understanding Antibiotic Resistance
In order to effectively combat antibiotic resistance, it is crucial to have a clear understanding of what it is and how it develops. This section will provide a comprehensive definition of antibiotic resistance and explore the various causes behind its emergence.
A. Definition of antibiotic resistance
Antibiotic resistance refers to the ability of bacteria to withstand the effects of antibiotics, rendering them ineffective in treating infections caused by these bacteria. In other words, the bacteria have developed the ability to survive and continue to multiply even in the presence of antibiotics.
This phenomenon occurs when bacteria undergo genetic changes or acquire resistance genes from other bacteria through horizontal gene transfer. These genetic changes enable the bacteria to produce enzymes that can inactivate antibiotics, alter the target site of antibiotics, or pump out the antibiotics from within the bacterial cells.
Antibiotic resistance poses a significant threat to public health as it limits the effectiveness of antibiotics, which are essential for treating various bacterial infections. It can lead to prolonged illnesses, increased healthcare costs, and even death in severe cases where infections become untreatable.
B. Causes of antibiotic resistance
There are several factors that contribute to the development and spread of antibiotic resistance. Understanding these causes is crucial for implementing effective strategies to combat this growing problem.
1. Overuse and misuse of antibiotics
One of the primary causes of antibiotic resistance is the overuse and misuse of antibiotics. This includes the unnecessary prescription of antibiotics for viral infections, which are not affected by antibiotics. Additionally, improper use of antibiotics, such as not completing the full course of treatment or using leftover antibiotics, can contribute to the development of resistance.
It is important for healthcare professionals to prescribe antibiotics judiciously and for individuals to follow their prescribed treatment plans strictly. This will help prevent the emergence and spread of antibiotic-resistant bacteria.
2. Evolution of bacteria
Bacteria have a remarkable ability to evolve and adapt to their environment. This includes developing resistance mechanisms against antibiotics. Through natural selection, bacteria that have acquired resistance genes or mutations that confer resistance have a survival advantage and can outcompete susceptible bacteria.
As a result, over time, the population of bacteria becomes dominated by resistant strains, leading to an increase in antibiotic resistance. This evolutionary process is accelerated by the widespread use of antibiotics, providing more opportunities for bacteria to develop resistance.
3. Lack of new antibiotic development
While bacteria continue to evolve and develop resistance, the development of new antibiotics has significantly slowed down in recent decades. This is partly due to the challenges and costs associated with discovering and developing new antibiotics.
As a result, there is a limited arsenal of effective antibiotics available to combat resistant bacteria. This further exacerbates the problem of antibiotic resistance, as healthcare providers are left with fewer treatment options for infections caused by resistant bacteria.
III. Impact of Antibiotic Resistance

Antibiotic resistance is a growing concern in the field of healthcare, with significant implications for mortality rates, the spread of infections, and healthcare costs. As an experienced healthcare professional with a deep understanding of this issue, I have witnessed firsthand the devastating impact of antibiotic resistance on patients and the healthcare system as a whole.
A. Increased mortality rates
One of the most alarming consequences of antibiotic resistance is the increase in mortality rates. When bacteria become resistant to antibiotics, it becomes much more difficult to treat infections effectively. This can lead to longer hospital stays, delayed treatment, and a higher risk of complications. In some cases, infections that were once easily treatable can become life-threatening, resulting in a higher number of deaths.
For example, I recall a patient who was admitted to the hospital with a simple urinary tract infection. Initially, the infection responded well to antibiotics, but over time, the bacteria developed resistance to the prescribed medication. Despite our best efforts, the infection spread to the kidneys, leading to sepsis and ultimately, the patient’s death. This tragic outcome could have been prevented if effective antibiotics were available.
B. Spread of infections
Antibiotic resistance also contributes to the spread of infections. When antibiotics fail to eliminate bacteria completely, the remaining bacteria can multiply and spread to other individuals. This is particularly concerning in healthcare settings, where patients with weakened immune systems are more susceptible to infections.
As a healthcare professional, I have witnessed the rapid spread of antibiotic-resistant infections within hospitals. In one instance, a patient with a resistant strain of Staphylococcus aureus (MRSA) unknowingly transmitted the bacteria to several other patients. Despite implementing strict infection control measures, the bacteria continued to spread, resulting in a significant outbreak. This highlights the urgent need for effective strategies to prevent and control the spread of antibiotic-resistant infections.
C. Higher healthcare costs
The financial burden of antibiotic resistance cannot be overlooked. Treating infections caused by antibiotic-resistant bacteria is often more expensive and time-consuming than treating infections that respond to standard antibiotics. The need for prolonged hospital stays, additional diagnostic tests, and more expensive medications significantly drives up healthcare costs.
Moreover, the economic impact extends beyond the direct costs of treatment. Antibiotic-resistant infections can lead to longer recovery times, increased disability, and lost productivity. This places a strain on individuals, families, and society as a whole.
For instance, a study conducted by the Centers for Disease Control and Prevention (CDC) estimated that antibiotic-resistant infections cost the U.S. healthcare system billions of dollars annually. These costs include both direct medical expenses and indirect costs associated with lost productivity. By addressing antibiotic resistance, we can not only save lives but also reduce the financial burden on healthcare systems.
IV. Common Antibiotic-Resistant Infections
Antibiotic resistance is a growing concern in the field of healthcare. As bacteria evolve and develop resistance to commonly used antibiotics, it becomes increasingly difficult to treat infections effectively. In this section, we will explore three common antibiotic-resistant infections: Methicillin-resistant Staphylococcus aureus (MRSA), Carbapenem-resistant Enterobacteriaceae (CRE), and Extended-spectrum beta-lactamase (ESBL) producing bacteria.
A. Methicillin-resistant Staphylococcus aureus (MRSA)
Methicillin-resistant Staphylococcus aureus, commonly known as MRSA, is a type of bacteria that has become resistant to many antibiotics, including methicillin and other penicillin-related antibiotics. MRSA infections can occur in various parts of the body, such as the skin, bloodstream, and lungs.
MRSA is often spread through direct contact with an infected person or by touching contaminated surfaces. It is commonly found in healthcare settings such as hospitals and nursing homes, but community-associated MRSA infections are also on the rise.
MRSA infections can range from mild skin infections, such as boils or abscesses, to more severe and life-threatening infections, such as pneumonia or bloodstream infections. Treatment options for MRSA infections are limited due to the bacteria’s resistance to many antibiotics. However, certain antibiotics, such as vancomycin and daptomycin, can still be effective in treating MRSA infections.
B. Carbapenem-resistant Enterobacteriaceae (CRE)
Carbapenem-resistant Enterobacteriaceae (CRE) are a group of bacteria that have developed resistance to carbapenem antibiotics, which are considered the last line of defense against multidrug-resistant infections. Enterobacteriaceae are a family of bacteria that includes common pathogens such as Escherichia coli (E. coli) and Klebsiella pneumoniae.
CRE infections are often associated with healthcare settings, particularly intensive care units and long-term care facilities. However, community-acquired cases have also been reported. CRE infections can cause a range of illnesses, including urinary tract infections, bloodstream infections, and pneumonia.
Treating CRE infections can be challenging due to limited treatment options. In some cases, combination therapy with multiple antibiotics may be necessary. However, the emergence of pan-resistant CRE strains, which are resistant to all available antibiotics, poses a significant threat to public health.
C. Extended-spectrum beta-lactamase (ESBL) producing bacteria
Extended-spectrum beta-lactamase (ESBL) producing bacteria are a group of bacteria that produce enzymes called beta-lactamases, which can break down and inactivate a broad range of antibiotics, including penicillins and cephalosporins. ESBL-producing bacteria are often found in the gastrointestinal tract and can cause infections in various parts of the body.
ESBL-producing bacteria are commonly associated with healthcare settings, but community-acquired cases have also been reported. Infections caused by ESBL-producing bacteria can be challenging to treat due to their resistance to multiple antibiotics. Treatment options may include carbapenems, which are less susceptible to the action of ESBL enzymes, or combination therapy with other antibiotics.
Preventing the spread of antibiotic-resistant infections is crucial in combating the rise of antibiotic resistance. Healthcare facilities should implement strict infection control measures, such as proper hand hygiene, appropriate use of antibiotics, and screening for antibiotic-resistant bacteria. Additionally, public awareness and education about the responsible use of antibiotics are essential in reducing the development and spread of antibiotic resistance.
V. Factors Contributing to Antibiotic Resistance

In order to fully understand the dangers of antibiotic resistance and how to combat it, it is crucial to examine the various factors that contribute to its development and spread. This section will delve into three key factors: agricultural use of antibiotics, inappropriate prescribing practices, and global travel and migration.
A. Agricultural use of antibiotics
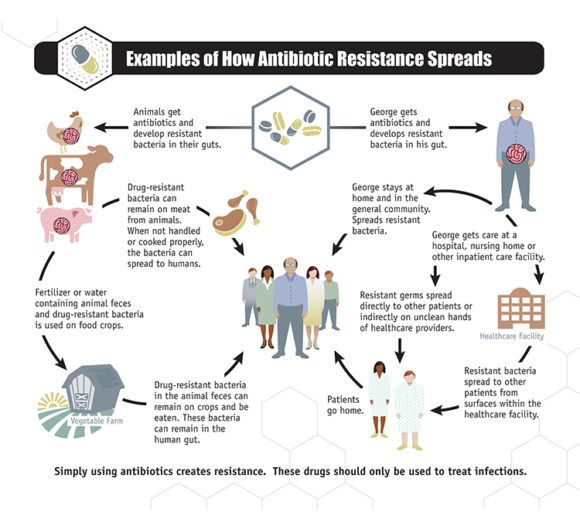
The use of antibiotics in agriculture has been a major contributor to the rise of antibiotic resistance. In many countries, antibiotics are routinely used in livestock farming to promote growth and prevent diseases. However, this widespread use has led to the emergence of antibiotic-resistant bacteria in animals, which can then be transmitted to humans through direct contact, consumption of contaminated food, or environmental contamination.
One of the main concerns is the use of antibiotics as a preventive measure rather than solely for the treatment of diagnosed infections. This practice not only promotes the development of antibiotic-resistant bacteria but also contributes to the overall overuse and misuse of antibiotics. It is essential for the agricultural industry to adopt more responsible and judicious use of antibiotics, such as implementing strict guidelines on their use, promoting alternative methods for disease prevention, and investing in research for sustainable farming practices.
B. Inappropriate prescribing practices
Inappropriate prescribing practices by healthcare professionals have also played a significant role in the emergence and spread of antibiotic resistance. This includes prescribing antibiotics when they are not necessary, prescribing broad-spectrum antibiotics instead of narrow-spectrum ones, and prescribing antibiotics for viral infections, which are not responsive to antibiotics.
There are several reasons behind these inappropriate prescribing practices. One is the pressure faced by healthcare professionals to meet patient expectations and demands for antibiotics, even when they may not be the most appropriate treatment. Another factor is the lack of awareness and education among both healthcare professionals and the general public about the appropriate use of antibiotics and the consequences of antibiotic resistance.
To address this issue, it is crucial to improve antibiotic stewardship programs, which promote the responsible use of antibiotics. This includes educating healthcare professionals about appropriate prescribing practices, implementing guidelines and protocols for antibiotic use, and raising awareness among the general public about the importance of using antibiotics judiciously.
C. Global travel and migration
The interconnectedness of our world through global travel and migration has also contributed to the spread of antibiotic resistance. People can carry antibiotic-resistant bacteria with them as they travel, and these bacteria can then be transmitted to others in different geographic locations.
In addition, medical tourism, where individuals travel to other countries for medical procedures, has become increasingly popular. This can pose a risk as individuals may acquire antibiotic-resistant infections during their medical procedures and bring them back to their home countries.
To address the challenges posed by global travel and migration, it is essential to strengthen international collaboration and coordination in monitoring and controlling the spread of antibiotic-resistant bacteria. This includes implementing surveillance systems to track the movement of antibiotic-resistant bacteria, sharing information and best practices among countries, and developing strategies to minimize the transmission of antibiotic-resistant infections through travel and migration.
VI. Strategies to Combat Antibiotic Resistance
Antibiotic resistance is a growing concern worldwide, as bacteria continue to develop resistance to commonly used antibiotics. To combat this issue, various strategies have been implemented to promote responsible antibiotic use, raise public awareness, develop new antibiotics, and explore alternative treatment options. In this section, we will discuss these strategies in detail.
A. Antibiotic stewardship programs
Antibiotic stewardship programs play a crucial role in combating antibiotic resistance. These programs aim to optimize antibiotic use by promoting appropriate prescribing practices, reducing unnecessary antibiotic use, and preventing the spread of antibiotic-resistant infections. They involve a multidisciplinary approach, including healthcare providers, pharmacists, infection control specialists, and patients.
One key component of antibiotic stewardship programs is education and training. Healthcare providers are educated on the principles of appropriate antibiotic use, including the importance of prescribing antibiotics only when necessary, selecting the right antibiotic based on the type of infection, and using the correct dosage and duration of treatment. This helps prevent the overuse and misuse of antibiotics, which can contribute to the development of antibiotic resistance.
Another important aspect of antibiotic stewardship programs is surveillance and monitoring. Healthcare facilities track antibiotic prescribing patterns, antibiotic resistance rates, and healthcare-associated infections. This data is used to identify areas of improvement, implement targeted interventions, and measure the impact of the program.
Additionally, antibiotic stewardship programs promote the use of diagnostic tests to guide antibiotic prescribing. Rapid diagnostic tests can identify the specific bacteria causing an infection and determine their susceptibility to antibiotics. This allows healthcare providers to prescribe the most effective antibiotic, reducing the risk of treatment failure and the development of antibiotic resistance.
B. Public education and awareness campaigns
Public education and awareness campaigns are essential in addressing antibiotic resistance. These campaigns aim to educate the general public about the appropriate use of antibiotics, the consequences of antibiotic resistance, and the importance of preventing infections through good hygiene practices.
One key message of these campaigns is the importance of completing the full course of antibiotics as prescribed by healthcare providers. Many people stop taking antibiotics once they start feeling better, which can contribute to the development of antibiotic resistance. Public education campaigns emphasize that completing the full course of antibiotics is necessary to ensure the complete eradication of the infection and prevent the survival of antibiotic-resistant bacteria.
These campaigns also highlight the role of individuals in preventing infections. Simple measures, such as regular handwashing, practicing safe food handling, and getting vaccinated, can help reduce the need for antibiotics and minimize the spread of antibiotic-resistant bacteria.
Furthermore, public education and awareness campaigns aim to dispel common misconceptions about antibiotics. Many people believe that antibiotics can treat viral infections, such as the common cold or flu, when in fact, antibiotics are ineffective against viruses. By raising awareness about the appropriate use of antibiotics, these campaigns help reduce unnecessary antibiotic prescribing and the emergence of antibiotic resistance.
C. Development of new antibiotics
The development of new antibiotics is crucial in the fight against antibiotic resistance. However, the discovery and development of new antibiotics have significantly declined in recent decades. This is partly due to the high cost and low profitability of antibiotic research and development compared to other therapeutic areas.
To incentivize the development of new antibiotics, various initiatives and policies have been implemented. These include government funding, research grants, and regulatory incentives to streamline the approval process for new antibiotics. Additionally, collaborations between academia, pharmaceutical companies, and research institutions are encouraged to accelerate the discovery and development of novel antibiotics.
Furthermore, efforts are being made to promote the responsible use of existing antibiotics to preserve their effectiveness. This includes promoting antibiotic stewardship programs, implementing guidelines for appropriate antibiotic use, and raising awareness among healthcare providers and the general public about the importance of preserving the effectiveness of existing antibiotics.
D. Alternative treatment options
In addition to the development of new antibiotics, exploring alternative treatment options is essential in combating antibiotic resistance. These alternative options include:
- Phage therapy: Phage therapy involves the use of bacteriophages, which are viruses that specifically target and kill bacteria. Phages can be used to treat bacterial infections that are resistant to antibiotics.
- Antibiotic combination therapy: Combining different antibiotics with different mechanisms of action can enhance their effectiveness and reduce the risk of resistance. This approach is particularly useful for treating multidrug-resistant infections.
- Antibiotic adjuvants: Adjuvants are substances that enhance the effectiveness of antibiotics. They can help overcome antibiotic resistance mechanisms in bacteria, making antibiotics more potent.
- Probiotics: Probiotics are beneficial bacteria that can help restore the natural balance of the microbiota and prevent the overgrowth of harmful bacteria. They can be used as a preventive measure to reduce the need for antibiotics.
These alternative treatment options offer promising strategies for combating antibiotic resistance. However, further research and clinical trials are needed to evaluate their safety, efficacy, and long-term effects.
VII. Importance of Proper Antibiotic Use
Proper antibiotic use is crucial in order to combat the growing problem of antibiotic resistance. In this section, we will explore the importance of completing the full course of antibiotics, avoiding unnecessary antibiotic use, and ensuring proper dosage and timing.
A. Completing the full course of antibiotics
One of the most important aspects of proper antibiotic use is completing the full course of treatment prescribed by your healthcare provider. Antibiotics are designed to kill bacteria or stop their growth, and it is essential to take them for the entire duration recommended, even if you start feeling better before the course is finished.
By completing the full course of antibiotics, you ensure that all the bacteria causing the infection are eradicated. If you stop taking the antibiotics prematurely, some bacteria may survive and develop resistance to the medication. This can lead to recurrent infections that are more difficult to treat.
It is important to note that the duration of antibiotic treatment varies depending on the type of infection and the specific antibiotic prescribed. Always follow your healthcare provider’s instructions and finish the entire course of antibiotics.
B. Avoiding unnecessary antibiotic use
Another crucial aspect of proper antibiotic use is avoiding unnecessary antibiotic use. Antibiotics are only effective against bacterial infections and are not effective against viral infections, such as the common cold or flu.
Using antibiotics for viral infections not only fails to treat the underlying cause of the illness but also contributes to the development of antibiotic resistance. When antibiotics are used unnecessarily, bacteria have more opportunities to develop resistance, making future infections harder to treat.
It is important to consult with your healthcare provider to determine whether antibiotics are necessary for your specific condition. They will be able to assess whether your symptoms are caused by a bacterial infection and prescribe the appropriate treatment.
C. Proper dosage and timing
Proper dosage and timing are essential for the effective use of antibiotics. It is important to take the prescribed dose at the recommended intervals to maintain a consistent level of the medication in your body.
Skipping doses or taking antibiotics at irregular intervals can result in suboptimal treatment and may contribute to the development of antibiotic resistance. It is crucial to follow the dosing instructions provided by your healthcare provider and set reminders if needed to ensure you take your antibiotics as prescribed.
In addition to proper timing, it is also important to take the correct dosage of antibiotics. Taking too little may not effectively treat the infection, while taking too much can increase the risk of side effects without providing additional benefits.
If you have any questions or concerns about the dosage or timing of your antibiotics, don’t hesitate to reach out to your healthcare provider for clarification.
VIII. Role of Healthcare Providers in Combating Antibiotic Resistance
As a healthcare provider, my role in combating antibiotic resistance is crucial. I have the responsibility to implement guidelines for antibiotic prescribing, conduct diagnostic testing to identify bacterial infections, and collaborate with public health agencies. By taking these actions, I can contribute to the fight against antibiotic resistance and ensure the effective use of antibiotics.
A. Implementing guidelines for antibiotic prescribing
One of the key ways healthcare providers can combat antibiotic resistance is by implementing guidelines for antibiotic prescribing. These guidelines help ensure that antibiotics are used appropriately and only when necessary. By following evidence-based guidelines, I can avoid overprescribing antibiotics and reduce the risk of antibiotic resistance.
When prescribing antibiotics, I consider several factors such as the type of infection, the severity of the illness, and the patient’s medical history. I also take into account the local antibiotic resistance patterns to make informed decisions. By prescribing the right antibiotic at the right dose and duration, I can minimize the development of antibiotic resistance.
Furthermore, I educate my patients about the importance of completing the full course of antibiotics as prescribed. Many patients tend to stop taking antibiotics once they feel better, which can contribute to the development of antibiotic resistance. By emphasizing the importance of adherence to treatment, I can help prevent the spread of resistant bacteria.
B. Diagnostic testing to identify bacterial infections
Accurate diagnosis is essential in the fight against antibiotic resistance. As a healthcare provider, I utilize diagnostic testing to identify bacterial infections and determine the most appropriate treatment. By differentiating between bacterial and viral infections, I can avoid unnecessary antibiotic prescriptions for viral illnesses.
I rely on various diagnostic tools such as laboratory tests, imaging studies, and clinical assessments to make an accurate diagnosis. These tests help me identify the specific bacteria causing the infection and determine its susceptibility to antibiotics. This information guides my treatment decisions, ensuring that antibiotics are targeted and effective.
Moreover, I stay updated on the latest advancements in diagnostic testing. New technologies, such as rapid molecular tests, can provide quick and accurate results, allowing for timely treatment decisions. By embracing these innovations, I can improve patient care and reduce the unnecessary use of antibiotics.
C. Collaboration with public health agencies
Collaboration with public health agencies is essential in the fight against antibiotic resistance. By working together, healthcare providers and public health agencies can implement strategies to prevent the spread of resistant bacteria and promote responsible antibiotic use.
I actively participate in surveillance programs that monitor antibiotic resistance patterns in my community. This information helps me make informed decisions regarding antibiotic prescribing and adjust treatment strategies accordingly. By sharing data with public health agencies, we can collectively identify emerging resistance trends and implement targeted interventions.
I also engage in educational initiatives organized by public health agencies to raise awareness about antibiotic resistance. Through these programs, I educate both healthcare professionals and the general public about the appropriate use of antibiotics, the consequences of antibiotic resistance, and the importance of infection prevention measures.
Furthermore, I collaborate with public health agencies to develop and implement antimicrobial stewardship programs. These programs aim to optimize antibiotic use, promote the use of narrow-spectrum antibiotics when appropriate, and prevent the spread of resistant bacteria within healthcare settings.
IX. Antibiotic Resistance and the Environment
Antibiotic resistance is a growing concern in today’s world. It not only affects human health but also has a significant impact on the environment. In this section, we will explore the issue of antibiotic pollution in water systems and its impact on wildlife and ecosystems.
A. Antibiotic Pollution in Water Systems
The widespread use of antibiotics in various sectors, including healthcare, agriculture, and veterinary medicine, has led to the contamination of water systems. When antibiotics are used, a portion of them is excreted by humans and animals and finds its way into rivers, lakes, and other water bodies through sewage systems and runoff.
This antibiotic pollution poses a serious threat to aquatic organisms and the overall health of the ecosystem. The presence of antibiotics in water systems can disrupt the natural balance of microorganisms, leading to the development of antibiotic-resistant bacteria.
Studies have shown that even at low concentrations, antibiotics can promote the growth of antibiotic-resistant bacteria in water bodies. These resistant bacteria can then spread to other organisms, including humans, through direct contact or consumption of contaminated water or seafood.
Furthermore, the presence of antibiotics in water systems can also have detrimental effects on non-target organisms. For example, aquatic plants and algae can be negatively affected, leading to a decline in biodiversity and ecological imbalance.
To combat antibiotic pollution in water systems, it is crucial to implement effective wastewater treatment processes that can remove antibiotics from the water. Additionally, promoting responsible use of antibiotics in healthcare, agriculture, and veterinary practices can help reduce the overall contamination of water bodies.
B. Impact on Wildlife and Ecosystems
The presence of antibiotics in the environment can have far-reaching consequences for wildlife and ecosystems. Wildlife, including fish, amphibians, and birds, can be exposed to antibiotics through direct contact with contaminated water or by consuming organisms that have been exposed to antibiotics.
One of the major concerns is the development of antibiotic resistance in wildlife populations. When animals are exposed to antibiotics, either through direct administration or environmental contamination, they can develop antibiotic-resistant bacteria in their gut or other parts of their body.
This antibiotic resistance can have serious implications for wildlife health and survival. It can lead to increased mortality rates, reduced reproductive success, and overall population decline. Additionally, antibiotic-resistant bacteria can also be transmitted from wildlife to humans, posing a public health risk.
Moreover, the presence of antibiotics in the environment can disrupt the delicate balance of ecosystems. Antibiotics can affect the composition and diversity of microbial communities, which play a crucial role in nutrient cycling and other ecological processes.
For example, the loss of certain microbial species due to antibiotic exposure can impact the breakdown of organic matter and nutrient availability, leading to cascading effects on other organisms in the ecosystem.
To mitigate the impact of antibiotic pollution on wildlife and ecosystems, it is important to promote sustainable practices in agriculture and aquaculture. This includes reducing the use of antibiotics in animal husbandry, implementing proper waste management strategies, and promoting the conservation of natural habitats.
